Ch4 Polar Or Nonpolar | Polarity describes the distribution of electrical charge around a here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity to predict which. Is ch4 polar or nonpolar. It has a colorless flammable gas with a musky and sweet odor. Ch4 polar mı apolar mı? Bir molekülün üzerindeki yük dağılımın simetrik olmaması demektir.
List molecules polar and non polar. There are, however, c2h2 and ch4, both of which are nonpolar. Is co2 polar or nonpolar? Polar protic vs polar aprotic vs nonpolar: Carbon dioxide molecules are nonpolar because they are highly symmetrical.
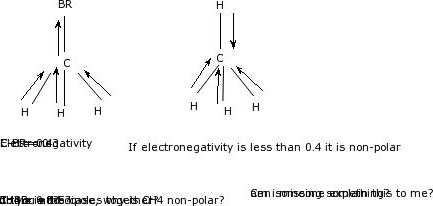
Nh3 is polar for the same reason as water. There are, however, c2h2 and ch4, both of which are nonpolar. Ch4 polar mı apolar mı? If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search. This type of molecule is called nonpolar molecule. C2h4 has two ch and four h molecules. Ch4 is not a polar molecule. About solvents in organic chemistry. Is ch4 polar or nonpolar. Ch4 is nonpolar because all of the nonpolar covalent bonds are spaced within a tetrahedral structure around the molecule. If you want to quickly find the word you want to search, use ctrl + f ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. One image shows that there are weak intermolecular interactions between nonpolar hexane. Well, moreover, the polar solvents possess molecules with polar bonds, and nonpolar solvents possess molecules with similar electronegativity values.
This distributes electron charge equally around the central carbon atom. Ch4 is not very much dissolved in water. A lot of students i talk to have questions about solvents, so i've these solvents have moderately higher dielectric constants than the nonpolar solvents (between 5 and 20). A polar molecule is a molecule containing polar bonds where the sum of all the bond's dipole moments is not zero. Although the bonds themselves are polar, the four bonds between carbon and fluorine cancel out one another, generating a nonpolar molecule.

The molecules that have atoms with equal electronegativity are nonpolar in nature because the equal charge why is ch4 nonpolar? Ch4 can constitute up to about 90 of natural gas depending on the the difference in electrostatic potential is also minimal giving an overall nonpolar molecule. As explained above, methane molecules are composed of 5 atoms ie; In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. Polar bonds form when there is a difference is ch3cl polar or nonpolar? Ch4 polar mı apolar mı? Methane ch 4 is a non polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms. Ch4, or methane, is the same as cf4 in this respect, as are many other molecules made of four halogens surrounding a carbon or silicon atom. 4 hydrogen atoms connected tetrahedrally with a. Ch4 and ccl4 have cancelling dipole moments and so are not polar. To find out the reason why the molecule is nonpolar, let's go through the factors that help us determine the polarity of the molecules. Ch2 does not exist as a molecule. Molecular polarity (polar or nonpolar).
Is brcl3 polar or nonpolar? Polarity describes the distribution of electrical charge around a here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity to predict which. Bir molekülün üzerindeki yük dağılımın simetrik olmaması demektir. Since the h is between b and c in terms on electronegativity values, their difference in electronegativity values is so. Ch4 is not a polar molecule.
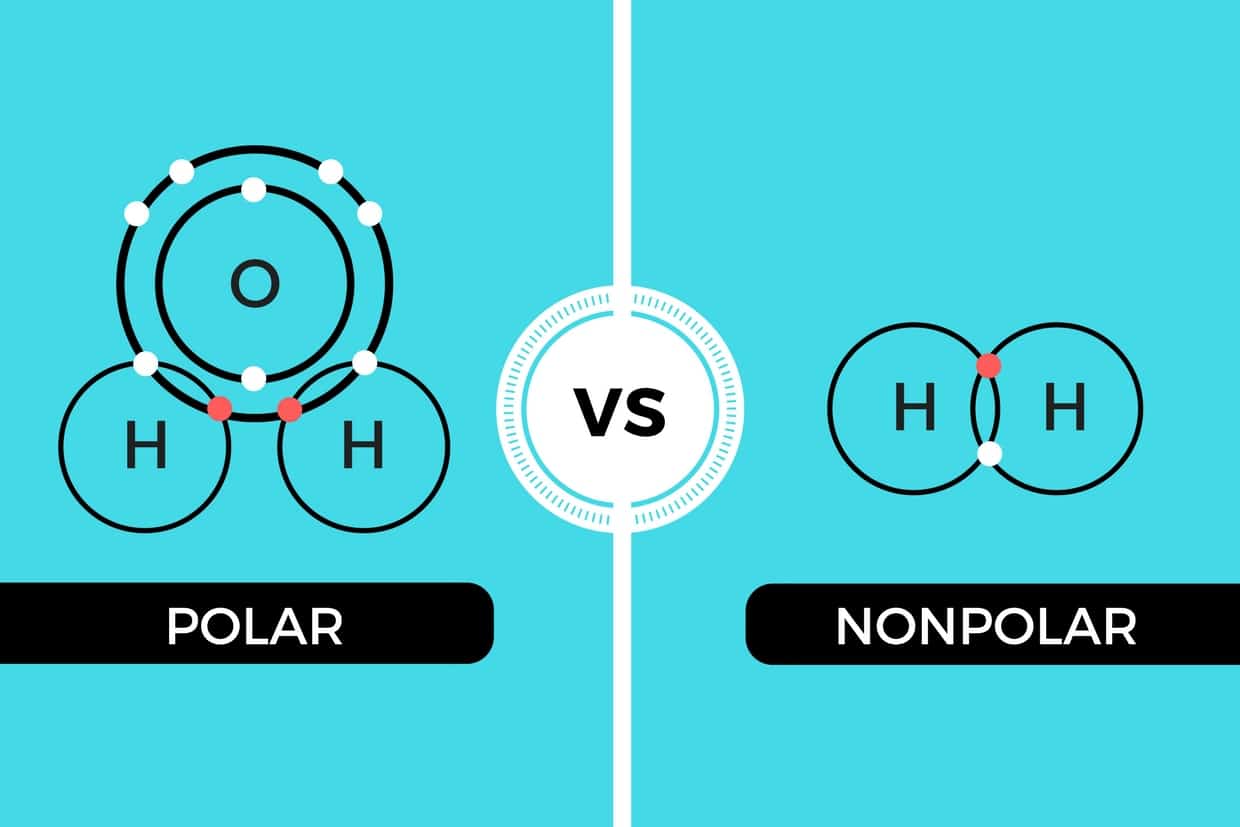
C2h4 has two ch and four h molecules. I'll tell you the polar or nonpolar list below. Bir molekülün üzerindeki yük dağılımın simetrik olmaması demektir. While it is true that the bonds between the carbon and each hydrogen atom are polar, the bond angles between. Methane or ch4 is a nonpolar molecule. Ch4 and ccl4 have cancelling dipole moments and so are not polar. Polar molecules vs nonpolar molecules. Since the h is between b and c in terms on electronegativity values, their difference in electronegativity values is so. To know the reason why it's nonpolar, read this article. List molecules polar and non polar. About solvents in organic chemistry. Is ch 4 polar or nonpolar? Methane ch 4 is a non polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms.
Ch4 Polar Or Nonpolar: Polar molecules vs nonpolar molecules.



Post a Comment